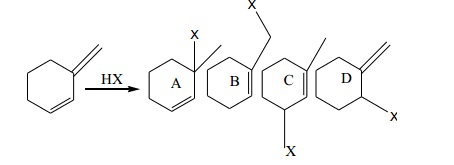
$\begin{array}{1 1}a)\;A\; and\; B\; are \;formed\; under\; thermodynamic\;control\\b)\; B\; and \;C\; are\; formed\; under \;thermodynamic\; control.\\c)\; A\; and\; C\; are\; formed\; under\; thermodynamic \;control.\\d)\; A\; and\; D \;are\; formed\; under\; thermodynamic\; control.\end{array}$