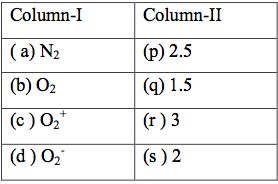
$\begin{array}{1 1}(1) (a ) \rightarrow (r), (b) \rightarrow (q) , (c ) \rightarrow (p) , (d) \rightarrow (s)\\(2) (a ) \rightarrow (r), (b) \rightarrow (p) , (c ) \rightarrow (s) , (d) \rightarrow (q)\\(3) (a ) \rightarrow (r), (b) \rightarrow (s) , (c ) \rightarrow (p) , (d) \rightarrow (q)\\(4) (a ) \rightarrow (r), (b) \rightarrow (s) , (c ) \rightarrow (q) , (d) \rightarrow (p)\end{array}$
