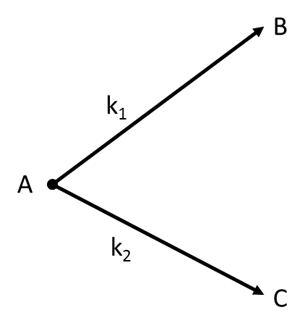
$\begin{array}{1 1}(a)\;\large\frac{[B]}{[c]}=\frac{k_2}{k_1}\;\normalsize at\; any\;time\;t\\(b)\;[B]=\large\frac{k_2[A_o]}{k_1+k_2}\normalsize(1-e^{-k_1t})\;at \;any\;time\;t\\(c)\;t_{1/2}=\large\frac{ln 2}{k_1+k_2}\\(d)\;k=\large\frac{k_1k_2}{k_1+k_2}\end{array}$
