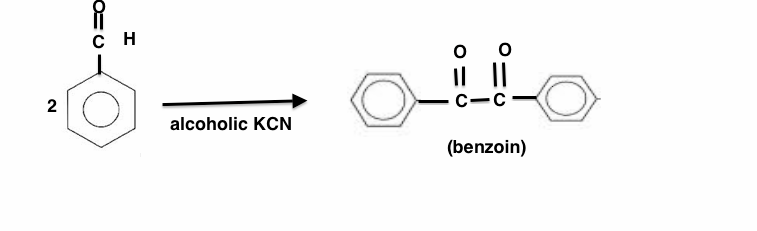
In the above reaction only $CN^-$ ions are effective because
$\begin{array}{1 1}(a)\;\text{Cyanide is a good nucleophile}\\(b)\;\text{Cyanide is an excellent electrophilic reagent}\\(c)\;\text{Cyanide is strong base}\\(d)\;\text{Cyanide is poisonous and its the base catalyst to force benzaldehyde to take a different course than cyanohydrin}\end{array}$
