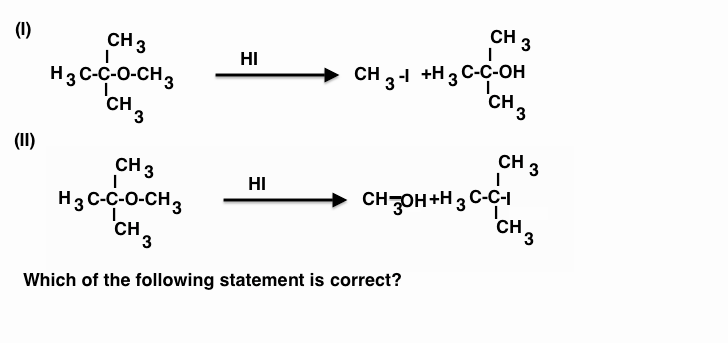
$\begin{array}{1 1}(a)\;\text{The reagent used in reaction (I) is anhydrous HI in ether and in reaction(II) is concentrated HI}\\(b)\;\text{The reagent used in both reactions (I) and (II) is ahydrous HI in ether}\\(c)\;\text{The reagent used both in reactions (I) is conc.HI and in reaction (II) is anhy.HI in ether}\\(d)\;\text{Above all statements are correct}\end{array}$