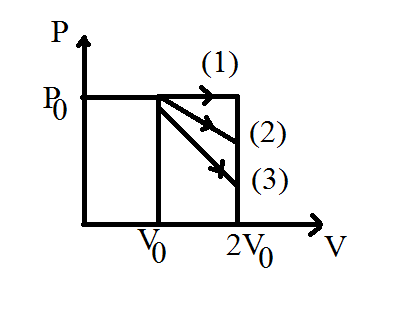
 $(a)\;\bigtriangleup u_{1} > \bigtriangleup u_{2} > \bigtriangleup u_{3}\qquad(b)\; \bigtriangleup u_{1} < \bigtriangleup u_{2} < \bigtriangleup u_{3}\qquad(c)\;\bigtriangleup u_{2} < \bigtriangleup u_{1} < \bigtriangleup u_{3}\qquad(d)\;\bigtriangleup u_{2} < \bigtriangleup u_{3} < \bigtriangleup u_{1}$
$(a)\;\bigtriangleup u_{1} > \bigtriangleup u_{2} > \bigtriangleup u_{3}\qquad(b)\; \bigtriangleup u_{1} < \bigtriangleup u_{2} < \bigtriangleup u_{3}\qquad(c)\;\bigtriangleup u_{2} < \bigtriangleup u_{1} < \bigtriangleup u_{3}\qquad(d)\;\bigtriangleup u_{2} < \bigtriangleup u_{3} < \bigtriangleup u_{1}$
