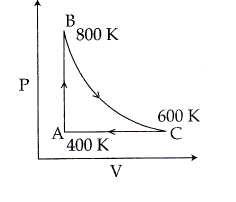
$\begin{array}{1 1}(A)\;\text{The change in internal energy in the process AB is – 350R} \\ (B)\;\text{The change in internal energy in the process BC is –500R} \\(C)\;\text{The change in internal energy in whole cyclic process is 250 R} \\(D)\;\text{The change in internal energy in the process CA is 700 R}\end{array} $
