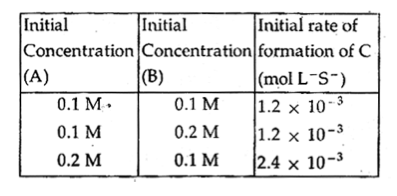
The rate law for the formation of C is
$\begin{array}{1 1}(A)\;\large\frac{dc}{dt}\normalsize= k[A][B]^2\\(B)\;\large\frac{dc}{dt}\normalsize= k[A]\\(C)\;\large\frac{dc}{dt}\normalsize= k[A][B]\\(D)\;\large\frac{dc}{dt}\normalsize= k[A]^2[B]\end{array} $
