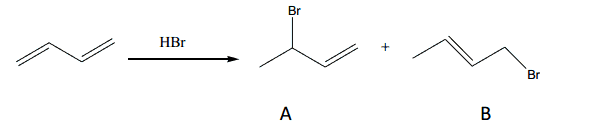
The incorrect statement about this reaction is: a)IntheintermediatethepositivechargeonthesecondarycarbonislargerthantheprimarycarbonresultinginmorerapidformationofAb)InBthedoublebondismorestabilizedbyhyperconjugationthaninA.c)Athighertemperature,bismainproduct.d)Underthekineticallycontrolledconditions,Aandbcaneasilyinterconvert.