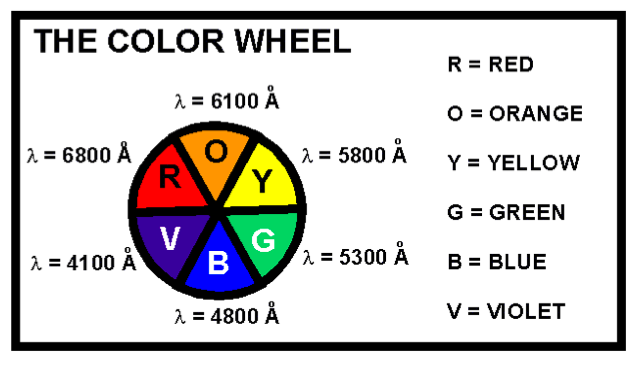
(a)Both chromium metal ions are paramagnetic with 3 unpaired electrons(b)Δoctfor[Cr(NH3)6]3+is calculated directly from the energy of yellow light(c)Δoctfor[Cr(OH2)6]3+is less thanΔoctfor[Cr(NH3)6]3+(d)A solution of [Cr(OH2)6]Cl3 transmits light with an approximate wavelength range of 4000 - 4200 angstroms
