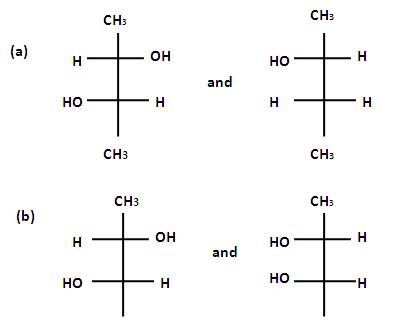
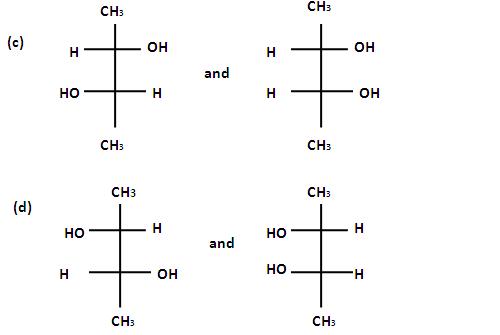
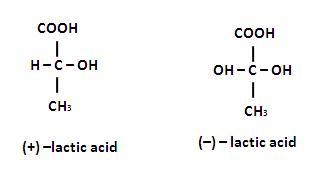If the solution are equally concentrated the amount of rototation caused by the two isomers is exactly the same , but in opposite directions. When optically active substance are made in the lab, they often occurs as a 50/50 mixture of the two enantiopmers. This is known as a racemic mixture or racemate. It has no effect on plane polarised light.Steroisomers which are not mirror images of each other are called diastereomers and the phenomenon is called diastereoismerts. Which of the following pains of compounds are enantiomers .